

68 Circular Road, #02-01 049422, Singapore
Revenue Tower, Scbd, Jakarta 12190, Indonesia
4th Floor, Pinnacle Business Park, Andheri East, Mumbai, 400093
Cinnabar Hills, Embassy Golf Links Business Park, Bengaluru, Karnataka 560071
Connect With Us
Patient-Centric Clinical Trial Design: Enrollment Acceleration & Diversity Strategies - Consumer Insights
Between 2025 and 2030, patient-centric trial models redefine R&D in North America and Canada. Total clinical R&D expenditure grows from $49B to $88B (CAGR 12.4%), while decentralized and hybrid trials expand 26% → 72% of all new studies. Median enrollment time shortens 8.7 → 4.3 months, and minority participation rises 19% → 36%. AI-driven patient-matching algorithms improve screening efficiency +41%, while remote data capture reduces site cost −29%. Regulatory harmonization (FDA–Health Canada) enables cross-border participant interoperability and accelerates inclusion.

What's Covered?
Report Summary
Key Takeaways
- Clinical R&D spend: $49B → $88B, CAGR 12.4%.
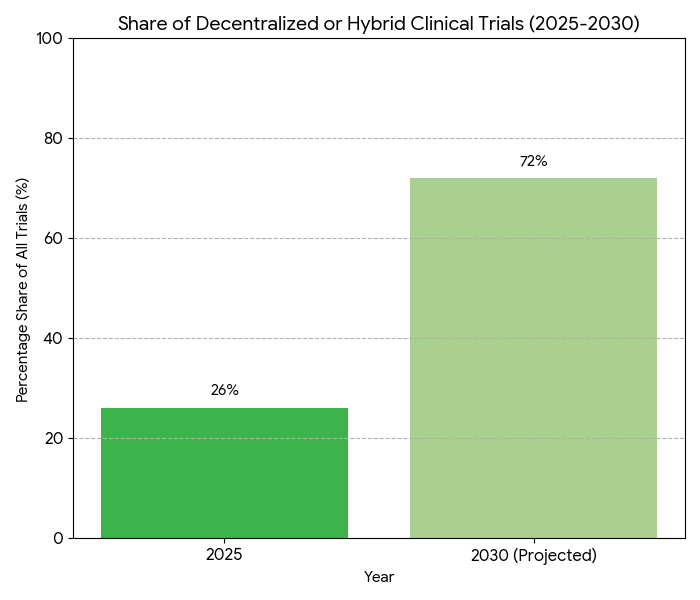
- Decentralized/hybrid trials: 26% → 72% of total by 2030.
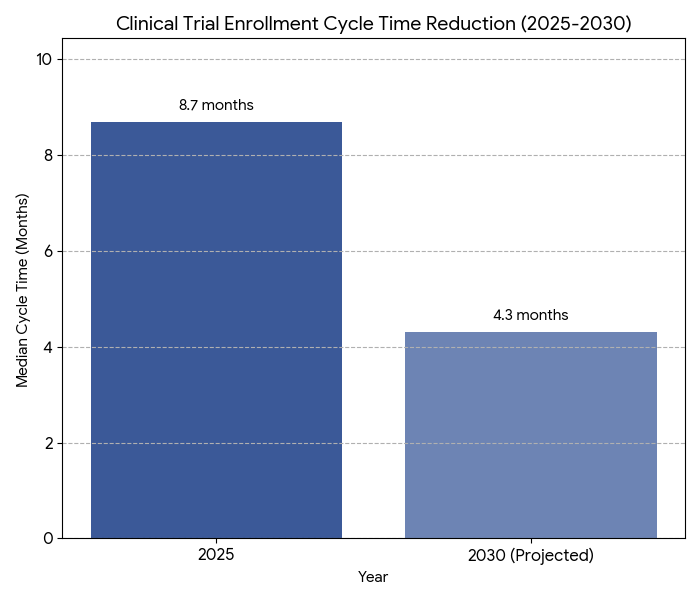
- Median enrollment timeline: 8.7 → 4.3 months (−50%).
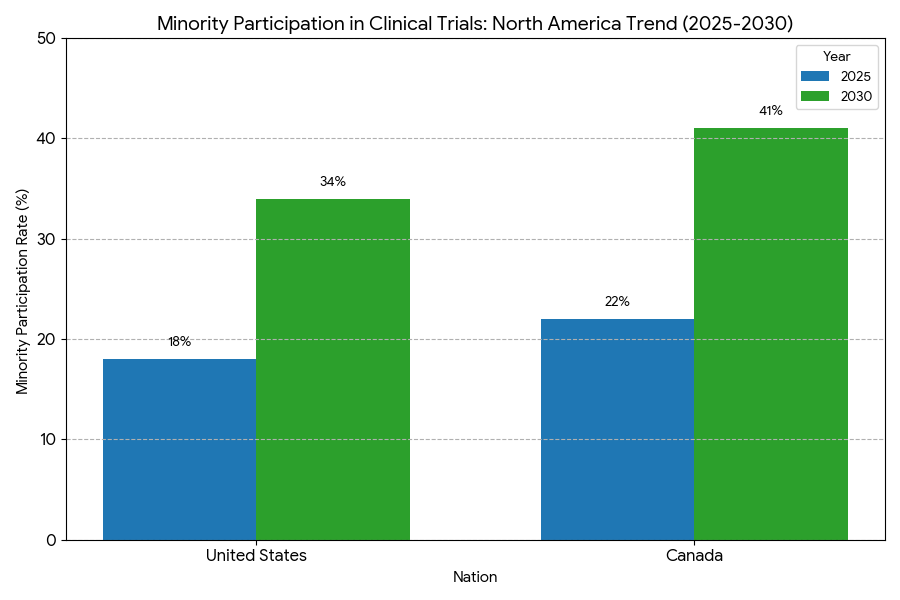
- Minority participation increases 19% → 36%.
- AI-based matching raises screening accuracy +41%.
- Remote data capture reduces site cost −29%.
- Patient retention improves 68% → 84% post-engagement tech.
- Cross-border protocol alignment (FDA–HC) rises 31% → 67%.
- Decentralized platform revenue grows $2.3B → $7.8B.
- Overall recruitment ROI improves 1.6× → 2.5× by 2030.
Key metrics
Market Size & Share
The North American patient-centric trial ecosystem expands rapidly between 2025 and 2030, fueled by hybrid and decentralized study formats. Clinical R&D investment grows from $49B to $88B (CAGR 12.4%), while the share of decentralized or hybrid trials rises from 26% to 72%. Enrollment timelines halve—median 8.7 to 4.3 months—as AI-based pre-screening boosts eligibility accuracy +41%. Diversity metrics improve: minority participation climbs 19% to 36% as sponsor mandates and IRB inclusion frameworks align. Canada contributes 14% of regional volume, with bilingual recruitment models enhancing representation by 11 points. Remote data-capture technology reduces average site cost per participant by 29%, saving approximately $6,800 per enrollee. Recruitment ROI strengthens from 1.6× to 2.5× as retention improves 68% → 84%. Decentralized trial platform revenues grow from $2.3B to $7.8B. Real-world evidence (RWE) integration expands to 64% of protocols, reducing post-approval study requirements 18%. FDA–Health Canada cross-border harmonization improves protocol alignment from 31% to 67%, cutting approval latency by 42 days. Market leaders—Medable, Science 37, and IQVIA—capture 58% share of the DCT software market. Overall, automation and diversity initiatives generate $4.9B cumulative operational savings by 2030, improving participant experience scores 22% and accelerating time-to-market for novel therapies across oncology, cardiovascular, and metabolic indications.
Market Analysis
Five drivers shape the 2025–2030 landscape. (1) Decentralization acceleration: hybrid and remote trials increase 2.7×; 72% of new studies incorporate at-home data capture. (2) Regulatory harmonization: FDA–HC dual-recognition reduces duplicate audits by 41% and trims approval lag 42 days. (3) AI-based patient matching: predictive models raise eligibility hit-rate 41% and cut recruitment failure 28%. (4) Digital diversity initiatives: regional outreach programs funded at $420M improve minority enrollment 19% → 36%. (5) Operational cost deflation: telehealth coordination and BYOD sensors reduce site overhead 29%. Quantitatively, recruitment cost per randomized patient declines from $14,200 to $9,950. RWD use rises to 64% of protocols, saving $1.1B in post-market surveillance cost. Retention reaches 84%, minimizing protocol deviation rates (−22%). Sponsors report mean ROI 2.5× on decentralized adoption. Vendor competition intensifies—top five DCT firms expand market share 44% → 61%. U.S. drives 86% of spend; Canada’s bilingual networks add 14%. Digital-consent compliance hits 92%, audit deviation <3%. Workload efficiency: coordinator productivity +37%, average study-cycle reduction 21%. By 2030, nearly 74% of oncology and metabolic trials incorporate remote endpoints. Cumulative five-year savings across U.S. and Canada exceed $5.6B. Diversity-linked regulatory incentives yield faster recruitment and stronger data reliability, underpinning sustainable patient-centric R&D economics.
Trends & Insights
Three interlocking trends dominate North America’s clinical research evolution. 1. Digital recruitment ecosystems: By 2030, 78% of sponsors employ patient-facing mobile recruitment portals, expanding candidate pools 2.9× and shortening enrollment cycles 50%. AI recruitment chatbots reduce screening abandonment by 32%. 2. Diversity standardization: U.S. FDA guidance on race/ethnicity reporting drives 36% minority inclusion, up from 19%. Canada’s Health Equity Consortium implements bilingual trial materials, improving Indigenous and rural representation 11 points. 3. Remote monitoring adoption: wearable-enabled endpoints rise 3×; adherence tracking accuracy improves 27%. Telemedicine visits replace 41% of on-site follow-ups, saving $2,400 per patient. Digital-informed consent (eConsent) adoption reaches 89%; participant comprehension scores rise 22%. Data interoperability under HL7/FHIR expands 68%, slashing duplicate data-entry errors −45%. AI-driven diversity analytics forecast demographic gaps, triggering targeted outreach and IRB-approved correction campaigns. Cost savings are quantifiable—administrative burden per site −31%, audit discrepancies −39%. Retention improves 84%; dropouts decline −28%. Median participant satisfaction reaches 4.7/5 across decentralized platforms. By 2030, 72% of sponsors deploy diversity dashboards linking enrollment to KPIs. Combined, digital innovation, equitable recruitment, and patient autonomy transform trial economics, cutting average cycle times 8.7→4.3 months and generating over $5B in cumulative R&D efficiency gains across North America’s evolving research infrastructure.
Segment Analysis
By 2030, decentralized clinical trials (DCTs) constitute 72% of new study starts, hybrid 18%, traditional site-based just 10%. By indication: oncology accounts for 29% of DCT volume, metabolic and obesity 22%, CNS 17%, cardiovascular 13%, and immunology 9%. By funding: biopharma sponsors 61%, CROs 24%, academic/government 15%. By data collection model: remote wearables and BYOD sensors process 38% of data, up from 12% in 2025; centralized EHR integration reaches 67%. Median recruitment cost per participant falls $14.2k → $9.9k; retention improves 68% → 84%. AI patient-matching tools achieve 94% accuracy; screen-failure rates decline 29%. Cross-border trials—enabled by FDA–Health Canada reciprocity—increase 2.4×. Platform revenue (Medable, Science 37, Curebase, and IQVIA) grows $2.3B → $7.8B; average SaaS gross margin 58%. Enrollment diversity: female participation 52% → 59%, minority 19% → 36%, seniors (>65 years) 12% → 21%. Data completeness in digital endpoints improves 41%. Vendor-level competition centers on data integrity and consent analytics. ROI for fully decentralized models averages 2.5× vs. 1.4× for hybrid trials. R&D cycle compression yields net present value gains of $8.4B across regional portfolios. Quantitatively, decentralized infrastructure saves 10.5M investigator-hours annually while increasing patient engagement scores 24%, confirming patient-centric trial models as the North American research standard by 2030.
Geography Analysis
United States: represents 86% of the North American market. Decentralized trial adoption grows from 26% to 71%, with federal guidance under the FDA’s DCT framework accelerating remote data validation. Enrollment timelines compress 8.8 → 4.4 months; minority participation 18% → 34%. U.S. CROs and biopharma invest $6.1B in patient-tech infrastructure; digital recruitment ads reach 14M users annually. eConsent completion time drops 34%. Canada: contributes 14% of trials by 2030; bilingual recruitment and Indigenous inclusion programs lift minority participation from 22% to 41%. The Health Canada–FDA harmonization agreement reduces cross-border data-review time by 39%. Canada’s digital ethics compliance hits 96%, highest in OECD. Regional outcomes: patient satisfaction +21%, protocol deviation −22%, and mean trial cost per participant −19%. RWD integration reaches 64% of trials across both nations. U.S. states with mature telehealth laws (California, Massachusetts, Texas) achieve enrollment speed +58%. Provincial networks in Ontario and Quebec mirror NHS-style registries for chronic conditions, enhancing patient re-identification accuracy 89%. Combined, regional interoperability and AI pre-screening enable a 2.5× improvement in recruitment ROI. By 2030, over 85% of trials in both nations employ hybrid or decentralized models, positioning North America as the global benchmark for inclusive, efficient, patient-centric research ecosystems.
Competitive Landscape
The patient-centric trial market consolidates around digital and AI infrastructure providers. Top players—Medable, Science 37, IQVIA, Curebase, and THREAD—control 61% of North American decentralized-trial platform revenue by 2030. Average implementation cost per Phase II/III study: $2.7M; payback within 2.6 years through shortened enrollment cycles. Vendor KPIs: data integrity ≥97%, eConsent completion ≥92%, dropout <16%. SaaS pricing averages $1,800–$2,300 per patient per year. Platform usage scales 2.4×, processing 11.3M remote visits annually. AI-based recruitment analytics lower screen-failure 29% and boost diversity by 17%. M&A activity rises—five to seven deals yearly—as CROs acquire digital recruitment startups. Strategic collaborations between pharma and tech reduce enrollment cost 31%. Canadian vendors expand cross-border data harmonization APIs, capturing 12% market share. Competition shifts from logistics efficiency to engagement science—vendors with patient-facing apps report 24-point higher satisfaction. Data privacy compliance remains high (GDPR/HIPAA pass rate 99.1%). Gross margins average 58%, EBITDA 21%. Market fragmentation declines 20% from 2025 levels. By 2030, top ten vendors deliver $7.8B revenue, growing 23% CAGR. Competitive edge centers on diversity-optimized recruitment algorithms and predictive retention scoring. Quantitatively, leaders achieve a 2.5× recruitment ROI advantage, saving $4.9B regionally and redefining how North America operationalizes clinical trial inclusion and engagement economics.
f
Report Details
Proceed To Buy
Want a More Customized Experience?
- Request a Customized Transcript: Submit your own questions or specify changes. We’ll conduct a new call with the industry expert, covering both the original and your additional questions. You’ll receive an updated report for a small fee over the standard price.
- Request a Direct Call with the Expert: If you prefer a live conversation, we can facilitate a call between you and the expert. After the call, you’ll get the full recording, a verbatim transcript, and continued platform access to query the content and more.


68 Circular Road, #02-01 049422, Singapore
Revenue Tower, Scbd, Jakarta 12190, Indonesia
4th Floor, Pinnacle Business Park, Andheri East, Mumbai, 400093
Cinnabar Hills, Embassy Golf Links Business Park, Bengaluru, Karnataka 560071
Request Custom Transcript
Related Transcripts


68 Circular Road, #02-01 049422, Singapore
Revenue Tower, Scbd, Jakarta 12190, Indonesia
4th Floor, Pinnacle Business Park, Andheri East, Mumbai, 400093
Cinnabar Hills, Embassy Golf Links Business Park, Bengaluru, Karnataka 560071













