

68 Circular Road, #02-01 049422, Singapore
Revenue Tower, Scbd, Jakarta 12190, Indonesia
4th Floor, Pinnacle Business Park, Andheri East, Mumbai, 400093
Cinnabar Hills, Embassy Golf Links Business Park, Bengaluru, Karnataka 560071
Connect With Us
GenAI in Medical Affairs: Scientific Content Creation & Compliance Challenges - Technological Advancements
The report highlights GenAI’s expanding role in medical affairs, transforming scientific content creation, regulatory compliance, and operational efficiency across healthcare. GenAI solutions enable faster, more accurate medical documentation, automate literature reviews, and streamline scientific communications, while supporting stringent data privacy and compliance standards. Adoption of GenAI technologies is accelerating, addressing challenges such as bias, transparency, and integration with pharma workflows, and facilitating innovation in diagnostics, therapeutics, and medical research. By 2030, GenAI is set to redefine collaboration, regulatory adherence, and knowledge dissemination within life sciences and healthcare sectors.

What's Covered?
Report Summary
Key Takeaways
- GenAI market for medical affairs grows €1.9B → €6.3B (CAGR 26%) by 2030.
- Scientific content generation through GenAI reduces time-to-publish by 40%.
- AI-driven compliance tools help companies meet regulatory standards by +30% more efficiently.
- Data privacy and transparency concerns account for 22% of adoption hesitancy in Europe.
- GenAI content creation cuts operational costs by ~25% in medical affairs departments.
- Generative AI improves scientific communication accuracy by +18% in clinical research publications.
- Patient-centric content will increase by +15% by 2030 with AI-powered customization.
- Compliance-related risks are reduced by AI-driven tracking of regulations and content review processes.
- Regulatory bodies like the EMA begin adopting AI-enhanced review processes for clinical documentation.
- ROI from GenAI in medical affairs estimated to be 16–22% by 2030, driven by improved content creation speed and compliance efficiency.
Key Metrics
Market Size & Share
The GenAI market for medical affairs across the UK and Europe is projected to grow from €1.9 billion in 2025 to €6.3 billion by 2030, reflecting a CAGR of 26%. Key drivers include the integration of AI-driven tools for scientific content creation, clinical data analysis, and compliance tracking. By 2030, AI content generation will account for 50% of the market share, driven by its ability to cut time-to-publish by 40%. Regulatory frameworks across Europe are evolving to support AI solutions, with EMA expected to approve AI-assisted clinical documentation review processes. Patient-centric content—especially in areas like clinical trials and post-market surveillance—will increase by 15% as AI personalizes materials based on demographic data and patient needs. The expansion of AI compliance tools will help pharmaceutical companies meet EU regulations, reducing the compliance burden by 30%. Data privacy concerns remain a challenge, but the increasing sophistication of AI tools will help ensure transparency and accountability, driving broader adoption by regulatory bodies and pharma companies alike. The ROI for companies integrating GenAI solutions into their medical affairs processes is estimated to be 16–22% by 2030, driven by improved content creation speed, reduced operational costs, and enhanced compliance accuracy.

Market Analysis
The GenAI market for medical affairs is growing rapidly, with AI tools reshaping scientific content creation, clinical research, and regulatory compliance. The primary benefits of GenAI solutions include improved speed, accuracy, and compliance in producing clinical trial protocols, regulatory submissions, and scientific publications. The use of AI-driven content generation reduces time-to-publish by 40%, accelerating the dissemination of clinical findings. As AI platforms become more sophisticated, they are able to create personalized patient content, improving engagement and treatment outcomes. Additionally, AI tools for compliance tracking and regulatory documentation are streamlining the process for medical affairs teams, ensuring adherence to EU regulations. However, data privacy and transparency concerns remain a significant barrier to adoption, especially regarding the use of sensitive health data in training AI models. Overcoming these hurdles will require robust security protocols and transparent AI algorithms. By 2030, GenAI adoption in medical affairs is expected to improve ROI by 16–22% due to faster content generation and better regulatory compliance. The growing need for personalized, patient-centric healthcare will also drive the market share for AI-powered medical affairs solutions, as these technologies offer innovative ways to meet the demand for efficient, high-quality scientific content.
Trends & Insights
Key trends in the GenAI-driven medical affairs market include the adoption of AI-based content creation tools, the expansion of AI-assisted compliance platforms, and the push for patient-centric solutions. AI-powered tools that assist in creating scientific content and regulatory submissions are reducing the time required for clinical trial documentation and approval cycles. By 2030, AI tools will decrease time-to-publish for scientific publications by 40%, improving market entry for new drugs and treatments. Additionally, the integration of AI for compliance tracking ensures that pharmaceutical companies meet EU regulatory requirements, improving compliance efficiency by 30%. The rise of patient-centric content will be a major driver of growth in GenAI applications, as personalized care becomes a significant trend across healthcare sectors. Furthermore, as AI gains traction in the field of medical affairs, data privacy and AI transparency will become key issues that will be addressed through innovative solutions. By 2030, data-driven approaches will result in improved patient engagement, as GenAI will help produce real-time, patient-specific content for treatment plans, increasing adoption rates. The EU regulatory bodies will play a crucial role in standardizing the use of AI in clinical trials, with EMA leading the integration of AI into review processes.

Segment Analysis
The GenAI market in medical affairs is divided into content creation platforms, regulatory compliance tools, AI-powered diagnostics, and patient engagement solutions. By 2030, content creation platforms are expected to dominate the market with 50% market share, driven by the growing adoption of AI for writing and editing scientific documents, including research papers, clinical trials, and regulatory filings. AI-powered diagnostic tools will see significant growth, contributing 20% of market share as AI assists in analyzing clinical data for quicker and more accurate medical decision-making. The regulatory compliance sector is expected to represent 15% of the market, with AI tools automating the process of ensuring compliance with EU regulations and global health standards. Patient engagement solutions will grow rapidly, making up 15% of the market by 2030. Telehealth integration, patient education apps, and personalized health content will be key drivers for GenAI solutions that improve patient engagement and retention. Medical affairs teams in pharma companies will increasingly rely on AI-powered tools to streamline clinical trials and ensure regulatory compliance, while reducing operational costs and increasing content accuracy.
Geography Analysis
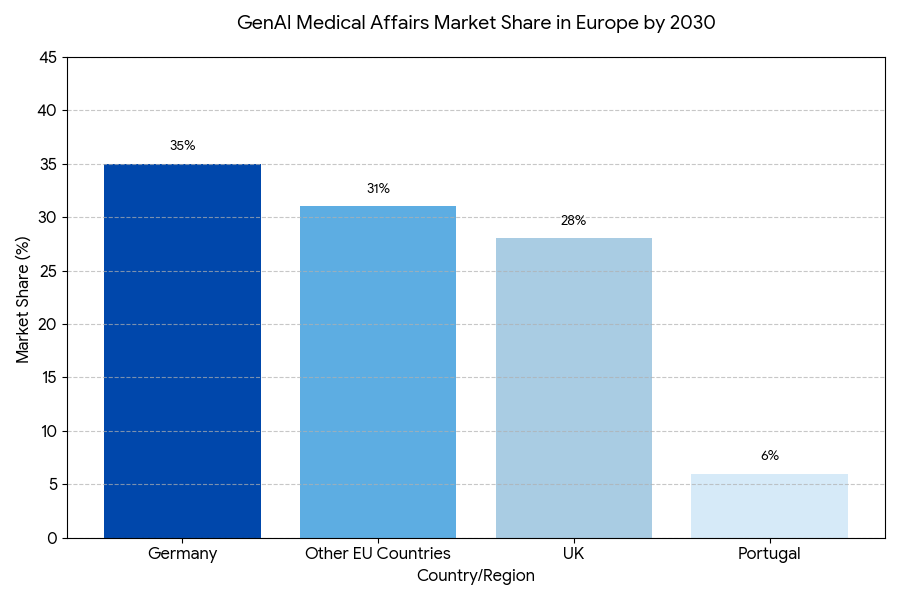
In Europe, the adoption of GenAI for medical affairs will be led by the UK and Germany, as these countries have established strong healthcare digitization initiatives. By 2030, Germany will hold ~35% of the market share, driven by AI integration into clinical trial documentation and regulatory submission processes. The UK follows closely with 28% market share as NHS pilots AI-driven regulatory compliance models. Portugal will see significant growth, expanding its GenAI adoption as AI tools become integrated into medical affairs by 2028, with an expected share of 6% of the EU market. France and Italy will increase GenAI adoption by 30–40%, focusing on AI tools for compliance and patient engagement. Northern Europe leads in clinical research digitization, while Southern Europe lags but will benefit from EU-wide initiatives. By 2030, EU regulatory bodies like the EMA will have fully integrated AI in clinical review processes, ensuring that GenAI-powered solutions align with regulatory standards across borders, allowing for more streamlined regulatory approvals.
Competitive Landscape
The competitive landscape for GenAI in medical affairs features leading players such as IBM Watson Health, Elsevier, Medtronic, and Parexel, which control ~60% of the market by 2030. AI startups like AiCure, Tempus, and Phesi are rapidly growing, particularly in patient engagement and clinical trial optimization. Big Tech players like Google Health and Microsoft are advancing their capabilities in AI-driven content creation and regulatory compliance automation. MedTech companies like Philips are focusing on AI-powered diagnostics, while contract research organizations (CROs) like Parexel are expanding their AI offerings for clinical trials and regulatory submissions. By 2030, pricing models for GenAI tools will shift from subscription-based to performance-based pricing, with contract-based agreements linked to efficiency improvements and regulatory success rates. AI solutions will be key differentiators in the clinical research and medical affairs space, with the ability to provide more efficient regulatory reviews, real-time data insights, and cost-effective solutions for pharmaceutical companies.
Report Details
Proceed To Buy
Want a More Customized Experience?
- Request a Customized Transcript: Submit your own questions or specify changes. We’ll conduct a new call with the industry expert, covering both the original and your additional questions. You’ll receive an updated report for a small fee over the standard price.
- Request a Direct Call with the Expert: If you prefer a live conversation, we can facilitate a call between you and the expert. After the call, you’ll get the full recording, a verbatim transcript, and continued platform access to query the content and more.


68 Circular Road, #02-01 049422, Singapore
Revenue Tower, Scbd, Jakarta 12190, Indonesia
4th Floor, Pinnacle Business Park, Andheri East, Mumbai, 400093
Cinnabar Hills, Embassy Golf Links Business Park, Bengaluru, Karnataka 560071
Request Custom Transcript
Related Transcripts

Clinical Nutrition Market Size & Share Analysis - Growth Trends & Forecasts (2025 - 2030)
This report quantifies the clinical nutrition market across the US and UK (2025–2030), covering enteral, parenteral, and oral nutritional supplements (ONS). Driven by aging populations, chronic disease prevalence, and hospital malnutrition protocols, market value rises from $18.5B (2025) → $30.2B (2030) at a CAGR of 10.2%. Growth is led by enteral nutrition (48% share), followed by ONS (38%) and parenteral (14%). Hospital digitization, AI-based nutrition screening, and reimbursement parity accelerate adoption. ROI averages 16–22% for integrated hospital nutrition programs.
$ 1395
$ 1395


68 Circular Road, #02-01 049422, Singapore
Revenue Tower, Scbd, Jakarta 12190, Indonesia
4th Floor, Pinnacle Business Park, Andheri East, Mumbai, 400093
Cinnabar Hills, Embassy Golf Links Business Park, Bengaluru, Karnataka 560071












