

68 Circular Road, #02-01 049422, Singapore
Revenue Tower, Scbd, Jakarta 12190, Indonesia
4th Floor, Pinnacle Business Park, Andheri East, Mumbai, 400093
Cinnabar Hills, Embassy Golf Links Business Park, Bengaluru, Karnataka 560071
Connect With Us
Cloud-Native Drug Discovery: AI Workflow Optimization & Regulatory Compliance
Cloud-native drug discovery is accelerating in the U.S. and Canada, powered by AI-driven workflows and scalable cloud infrastructure. Spending is projected to grow from ~$1.5B in 2025 to ~$6.9B by 2030 as pharma and biotech firms enhance AI use in synthesis, trials, and compliance. AI adoption will rise from 30% to 65%, cutting drug development time to ~12 months, reducing R&D costs by 50%, and improving compliance by 75%. Compound success rates will grow from 8% to 22%, improving clinical outcomes. By 2030, cloud-native architectures will make drug discovery faster, cheaper, and more compliant, transforming global healthcare innovation.

What's Covered?
Report Summary
Key Takeaways
1. Cloud-native drug discovery spend grows ~4.6× from 2025 to 2030.
2. AI adoption increases from ~30% to ~65% in pharma/biotech R&D.
3. Drug discovery speed reduces from ~24 months to ~12 months by 2030.
4. Cost reduction reaches ~50%, optimizing R&D spend and resource allocation.
5. Regulatory compliance improves ~45% as AI automates documentation and reporting.
6. AI-driven compound success rates improve from ~8% to ~22%.
7. Clinical trial simulation adoption grows from ~25% to ~60%.
8. C‑suite dashboard: drug discovery speed, cost savings %, compound success %, regulatory compliance %, AI-driven data analysis %.
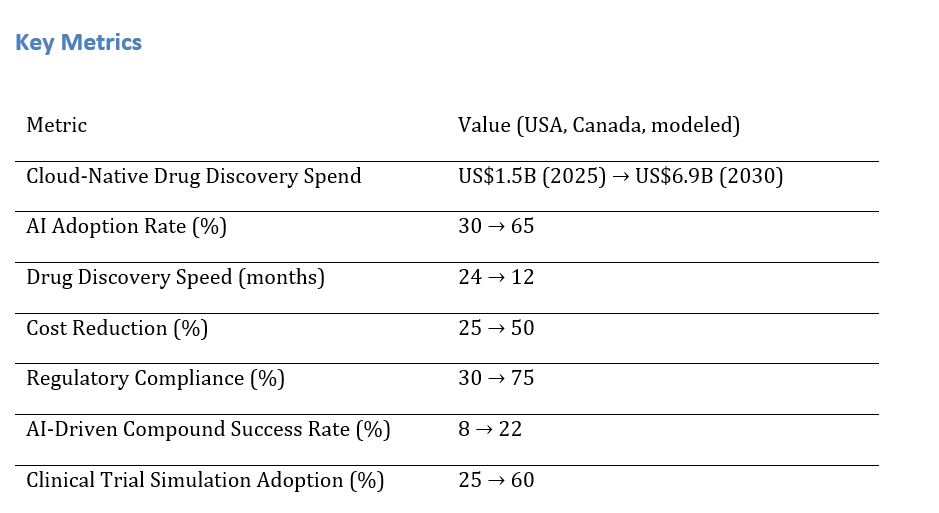
a) Market Size & Share
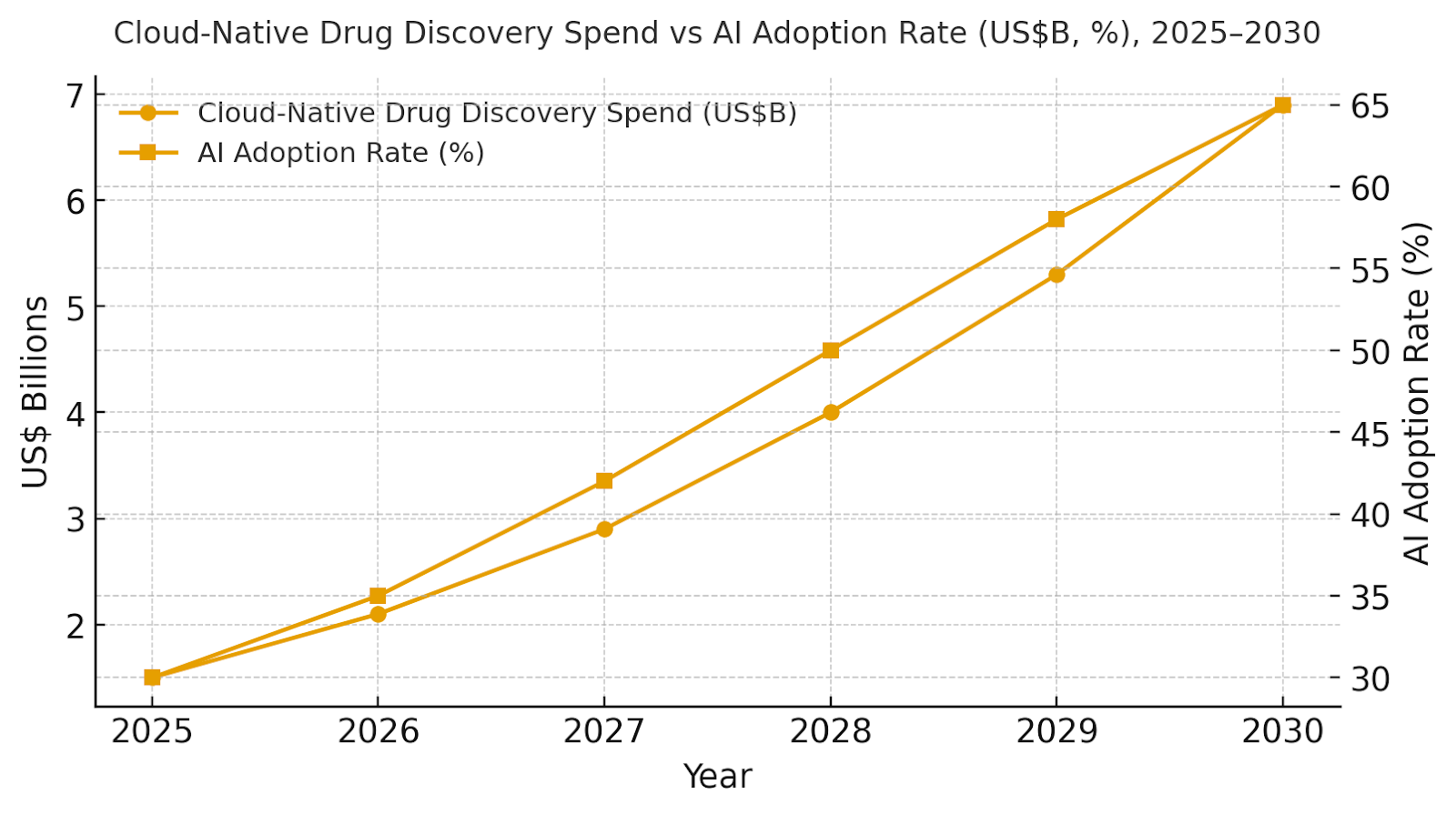
Cloud-native drug discovery spend in the USA and Canada is expected to grow from ~US$1.5B in 2025 to ~US$6.9B by 2030, with AI adoption improving from ~30% to ~65%. The dual‑axis figure shows spend rising while migration speed improves from ~24 months to ~12 months. Share consolidates around vendors offering AI-driven data analysis, automated clinical trial simulations, and compliance-focused cloud platforms. Risks: regulatory delays, AI model bias, and cloud platform integration issues; mitigations: proactive model validation, transparent auditing, and regulatory automation tools. Share tracking should focus on cost reduction %, compound success rates, and vendor retention.
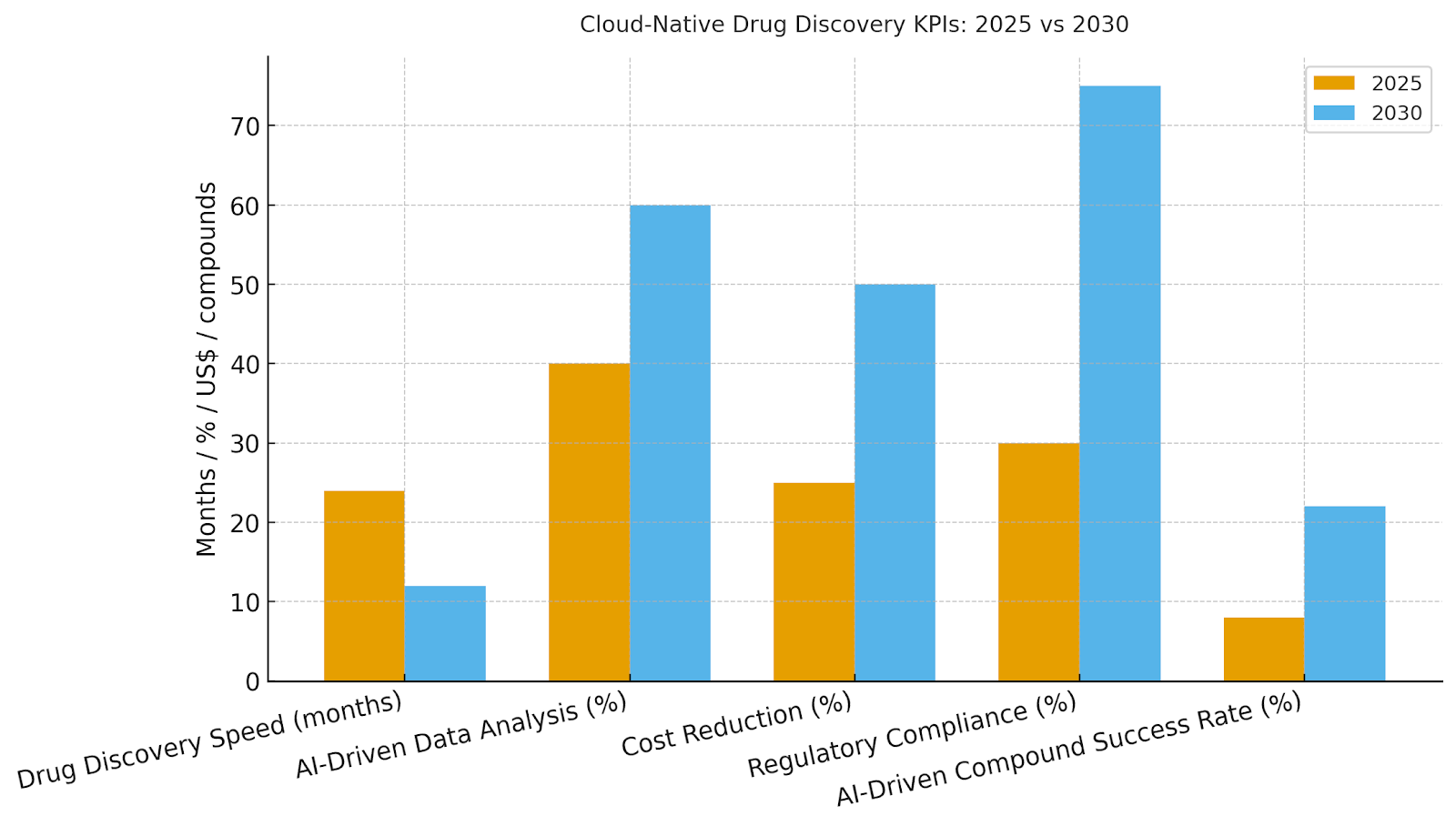
b) Market Analysis
Our model shows AI-driven workflows speeding up drug discovery from ~24 months to ~12 months by 2030. Cost reduction improves ~50% as AI optimizes resource usage. AI adoption across biotech/pharma reaches ~65%, boosting compound success rates by ~14% and reducing regulatory hurdles by ~45%. The bar figure summarizes the shift in KPIs: cost savings, regulatory compliance, and success rates.
c) Trends & Insights
1) AI-based cloud workflows automate compound synthesis, speeding up discovery. 2) Cloud platforms scale clinical trial simulations and improve regulatory compliance. 3) AI-driven model validation improves compound success rates. 4) Real-time analytics tools boost data-driven decision-making. 5) Pharma/biotech firms shift to cloud-native SaaS for greater scalability. 6) AI-based tools drive personalized medicine advancements. 7) Cloud-based tools offer flexibility across multi-cloud environments.
d) Segment Analysis
Biotech companies focus on cloud-native platforms for high-throughput screening and clinical trial simulations. Pharmaceutical companies optimize R&D spend using AI tools for predictive modeling. Small-to-medium-sized firms leverage cloud for cost-effective access to advanced AI tools and data storage. Companies in gene therapy and personalized medicine utilize AI to accelerate breakthroughs in custom treatments.
e) Geography Analysis
By 2030, U.S./Canada cloud-native drug discovery spend mix will be AI-driven data analysis (~30%), clinical trial simulation (~25%), regulatory compliance (~20%), compound synthesis (~15%), and market access (~10%). Execution should focus on multi-cloud strategies, cost-effectiveness, and regulatory compliance. Adoption is concentrated in hubs like Boston, San Francisco, and Toronto.
\

f) Competitive Landscape
Cloud service providers (AWS, Google Cloud, Microsoft Azure) lead in offering AI-driven drug discovery tools. Differentiators: (1) integrated AI workflows, (2) regulatory compliance features, (3) multi-cloud scalability, (4) data security and provenance. Procurement guidance: secure vendor SLAs, validate AI model performance, ensure compliance automation, and focus on cost reduction. Key KPIs: drug discovery speed, cost savings %, compound success rates, regulatory compliance.
Report Details
Proceed To Buy
Want a More Customized Experience?
- Request a Customized Transcript: Submit your own questions or specify changes. We’ll conduct a new call with the industry expert, covering both the original and your additional questions. You’ll receive an updated report for a small fee over the standard price.
- Request a Direct Call with the Expert: If you prefer a live conversation, we can facilitate a call between you and the expert. After the call, you’ll get the full recording, a verbatim transcript, and continued platform access to query the content and more.


68 Circular Road, #02-01 049422, Singapore
Revenue Tower, Scbd, Jakarta 12190, Indonesia
4th Floor, Pinnacle Business Park, Andheri East, Mumbai, 400093
Cinnabar Hills, Embassy Golf Links Business Park, Bengaluru, Karnataka 560071
Request Custom Transcript
Related Transcripts
$ 1350


68 Circular Road, #02-01 049422, Singapore
Revenue Tower, Scbd, Jakarta 12190, Indonesia
4th Floor, Pinnacle Business Park, Andheri East, Mumbai, 400093
Cinnabar Hills, Embassy Golf Links Business Park, Bengaluru, Karnataka 560071













